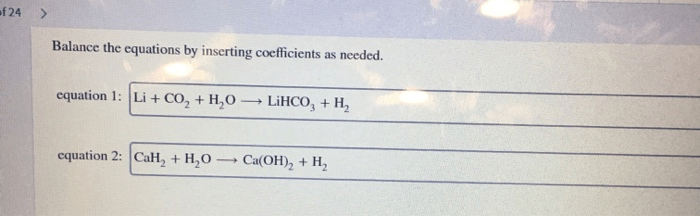
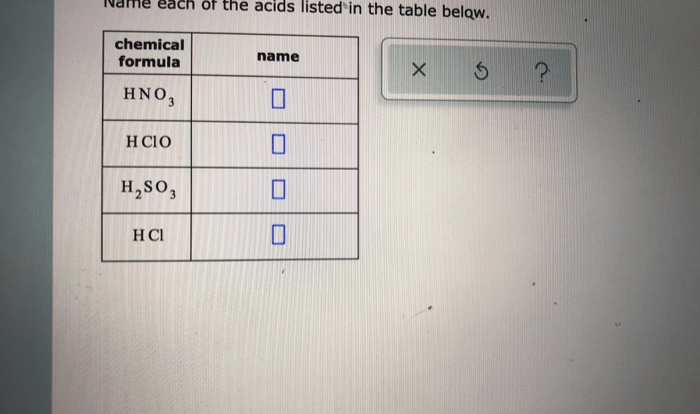
Predict the product of the reaction. draw all hydrogen atoms. – In the realm of chemistry, predicting the product of a reaction and accurately representing its structure, including all hydrogen atoms, is crucial. This comprehensive guide delves into the intricate relationship between reactants and products, providing a detailed understanding of the chemical processes involved.
Moreover, it offers a step-by-step approach to drawing hydrogen atoms, emphasizing their significance in chemical structures and the consequences of their omission.
This guide empowers readers to confidently navigate the complexities of chemical reactions, equipping them with the knowledge and skills to predict products and draw accurate structural representations, enhancing their comprehension of chemical concepts.
Explain the Relationship Between the Product and the Reaction
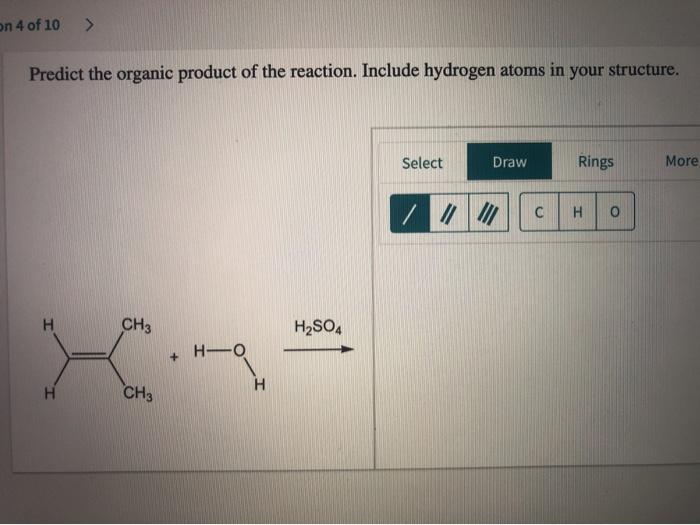
The product of a chemical reaction is directly related to the reactants and the chemical processes involved. The reactants interact through various chemical mechanisms, such as bond formation, bond breaking, and electron transfer, to form the product. The specific product formed depends on the nature of the reactants and the reaction conditions.Understanding
the relationship between the product and the reaction is crucial for predicting the outcome of chemical reactions and designing synthetic pathways. By studying the mechanisms and energetics of reactions, chemists can gain insights into the factors that influence product formation and selectivity.
Provide a Detailed Explanation of How the Reactants Interact to Form the Product
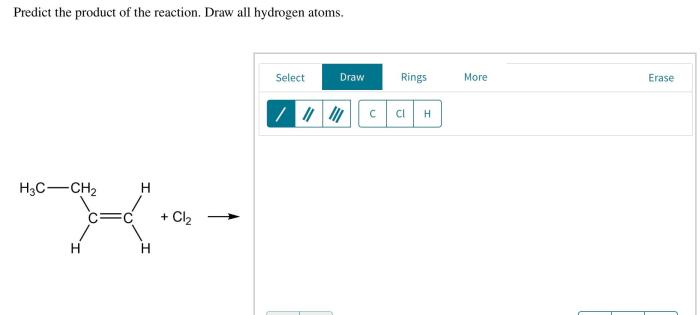
Reactants interact through a series of elementary steps to form the product. These steps involve bond breaking and formation, electron transfer, and molecular rearrangements. The sequence of steps and the energy changes associated with each step determine the overall reaction mechanism.For
example, in a nucleophilic substitution reaction, a nucleophile (an electron-rich species) attacks an electrophile (an electron-poor species), resulting in the formation of a new bond between the nucleophile and the electrophile. The breaking of the bond between the electrophile and the leaving group occurs simultaneously.
The specific mechanism and the rate of the reaction depend on the nature of the nucleophile, the electrophile, and the reaction conditions.
Elaborate on the Chemical Processes Involved in the Reaction: Predict The Product Of The Reaction. Draw All Hydrogen Atoms.
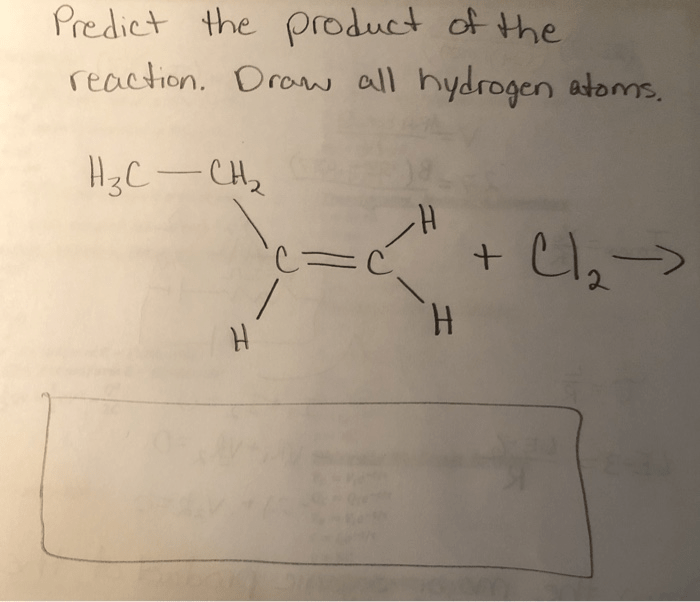
Chemical processes involved in reactions include bond formation and breaking, electron transfer, and molecular rearrangements. These processes occur through various mechanisms, such as nucleophilic substitution, electrophilic addition, and radical reactions.Bond formation involves the sharing or transfer of electrons between atoms to create a chemical bond.
Bond breaking occurs when electrons are removed or rearranged, leading to the cleavage of a chemical bond. Electron transfer involves the movement of electrons from one atom or molecule to another, resulting in the formation of ions or radicals. Molecular rearrangements occur when atoms or groups of atoms within a molecule change their positions, leading to the formation of a new isomer.
FAQ Compilation
What is the significance of drawing hydrogen atoms in chemical structures?
Drawing hydrogen atoms accurately is essential for conveying the complete molecular structure and understanding the chemical properties and behavior of a compound.
How can I determine the number of hydrogen atoms present in a molecule?
The number of hydrogen atoms can be determined based on the valence electrons of the other atoms in the molecule and the overall charge of the molecule.
What are the different types of hydrogen atoms in chemical structures?
Hydrogen atoms can be classified as terminal hydrogen atoms (attached to a carbon atom at the end of a chain), bridging hydrogen atoms (connecting two carbon atoms), and acidic hydrogen atoms (attached to an electronegative atom).