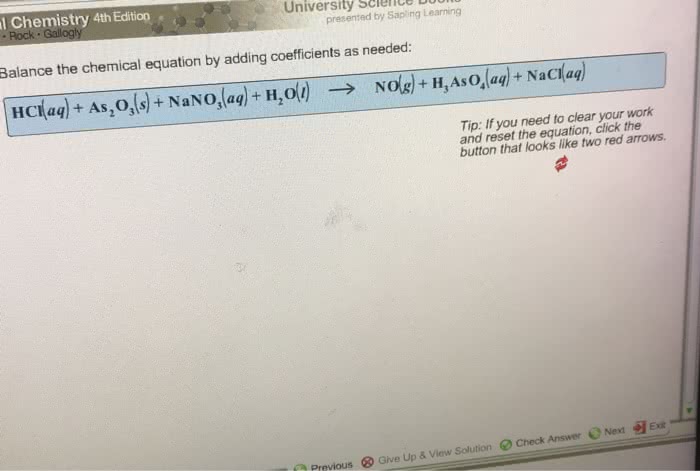
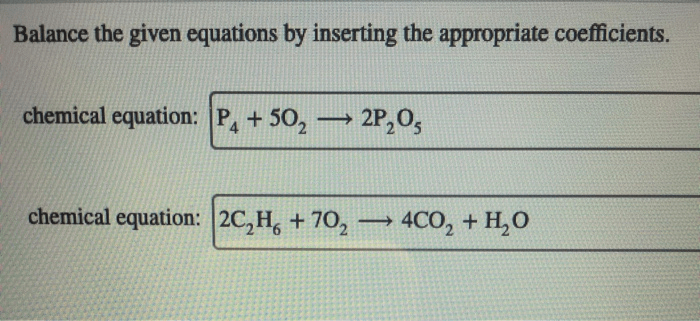
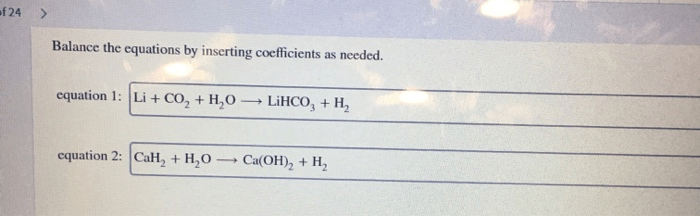
Balance the equations by inserting coefficients as needed – Balancing chemical equations is a crucial skill in chemistry, and inserting coefficients is the key to achieving this. This comprehensive guide will delve into the methods, techniques, and applications of balancing equations, empowering you to navigate the complexities of chemical reactions with precision and confidence.
The concept of coefficients plays a pivotal role in balancing equations, as they adjust the stoichiometric ratios of reactants and products to ensure the conservation of mass and charge. Understanding their significance is paramount to mastering the art of equation balancing.
1. Introduction
Balancing chemical equations is a crucial step in chemistry that ensures the conservation of mass and charge. It involves adjusting the stoichiometric coefficients in front of each chemical species to make the number of atoms of each element equal on both sides of the equation.
Coefficients represent the relative number of molecules or moles of each reactant and product involved in a chemical reaction. Balancing equations helps determine the exact amounts of reactants and products required or produced in a reaction, allowing for accurate predictions and calculations.
2. Methods for Balancing Equations
Step-by-Step Guide, Balance the equations by inserting coefficients as needed
Balancing equations can be done using various methods, including:
- Inspection: By examining the equation and making adjustments to the coefficients until the atoms of each element are balanced.
- Half-Reaction Method: By splitting the equation into oxidation and reduction half-reactions and balancing each half-reaction separately.
- Oxidation Number Method: By assigning oxidation numbers to each atom and adjusting the coefficients to ensure the total oxidation number change is zero.
3. Examples of Balancing Equations
| Unbalanced Equation | Balanced Equation |
|---|---|
| H2 + O2 → H2O | 2H2 + O2 → 2H2O |
| CH4 + 2O2 → CO2 + 2H2O | CH4 + 2O2 → CO2 + 2H2O |
| Fe + HCl → FeCl2 + H2 | Fe + 2HCl → FeCl2 + H2 |
4. Common Mistakes and Tips
- Not counting atoms carefully.
- Changing subscripts instead of coefficients.
- Balancing equations in steps.
To avoid these mistakes, it is important to:
- Count atoms meticulously.
- Adjust coefficients only.
- Balance the equation one element at a time.
5. Advanced Techniques for Balancing Equations
For complex equations, advanced techniques such as the half-reaction method and the oxidation number method can be used. These methods involve assigning oxidation numbers to atoms and manipulating the coefficients to ensure that the total oxidation number change is zero.
6. Applications of Balanced Equations: Balance The Equations By Inserting Coefficients As Needed
- Determining stoichiometry: Predicting the exact amounts of reactants and products involved in a reaction.
- Predicting reaction outcomes: Foreseeing the products and their quantities based on the balanced equation.
- Understanding reaction mechanisms: Identifying the steps involved in a chemical reaction and the role of each reactant and product.
Question & Answer Hub
What is the purpose of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element is the same on both sides of the equation, reflecting the conservation of mass in chemical reactions.
How do coefficients affect the stoichiometry of a reaction?
Coefficients adjust the stoichiometric ratios of reactants and products, determining the relative amounts of each substance involved in the reaction.
What are some common mistakes made when balancing equations?
Common mistakes include changing the chemical formulas of reactants and products, failing to balance polyatomic ions as a whole, and neglecting to account for charges when balancing redox reactions.