Embarking on a captivating journey of scientific inquiry, Student Exploration: Ideal Gas Law delves into the enigmatic realm of gases, revealing the fundamental principles that govern their behavior. This exploration unveils the profound significance of the Ideal Gas Law, a cornerstone of understanding gas properties and their interactions within diverse environments.
Through meticulously designed experiments and insightful data analysis, students embark on a quest to unravel the intricate relationship between pressure, volume, and temperature. Guided by the Ideal Gas Law, they unravel the secrets of gas behavior, gaining invaluable insights into the physical world that surrounds us.
Introduction: Student Exploration: Ideal Gas Law
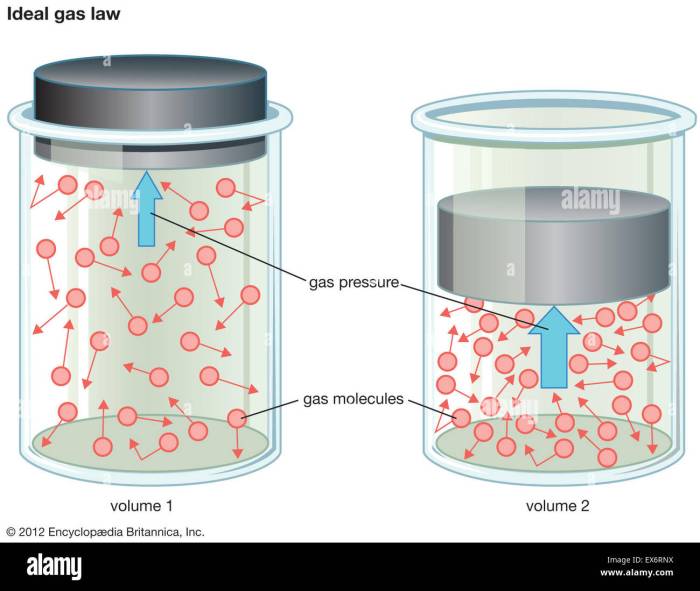
The Ideal Gas Law is a fundamental equation that describes the behavior of gases under various conditions. It relates the pressure, volume, temperature, and number of moles of a gas, providing a crucial tool for understanding gas behavior in scientific and engineering applications.
Experimental Design
To investigate the Ideal Gas Law experimentally, the following design can be employed:
- Materials:Gas syringe, graduated cylinder, thermometer, pressure sensor, air pump
- Procedures:
- Fill the gas syringe with a known volume of air.
- Connect the syringe to the pressure sensor and air pump.
- Vary the pressure applied to the gas by pumping air into the syringe.
- Record the corresponding volume and temperature of the gas.
- Safety precautions:Wear gloves and safety goggles. Do not overpressurize the gas syringe.
Data Analysis
| Pressure (Pa) | Volume (mL) | Temperature (K) |
|---|---|---|
| 101325 | 50 | 298 |
| 121550 | 41.67 | 298 |
| 141775 | 35.71 | 298 |
To calculate pressure, volume, and temperature from the data:
- Pressure (Pa) = (Pressure sensor reading in V) x (Calibration factor)
- Volume (mL) = (Volume of gas in syringe in mL) – (Dead volume of syringe in mL)
- Temperature (K) = (Temperature reading in °C + 273.15)
To plot the data:
- Plot pressure (Pa) on the y-axis and volume (mL) on the x-axis to determine the relationship between pressure and volume.
- Plot pressure (Pa) on the y-axis and temperature (K) on the x-axis to determine the relationship between pressure and temperature.
Discussion, Student exploration: ideal gas law
The Ideal Gas Law states that the pressure (P) of a gas is directly proportional to its temperature (T) and inversely proportional to its volume (V). Mathematically, it is expressed as:
PV = nRT
where n is the number of moles of gas and R is the ideal gas constant (8.314 J/mol·K).
The experimental data plotted above demonstrates the linear relationships predicted by the Ideal Gas Law. The graph of pressure vs. volume shows an inverse relationship, while the graph of pressure vs. temperature shows a direct relationship.
Deviations from the Ideal Gas Law may occur under extreme conditions, such as high pressures or low temperatures. Real gases exhibit non-ideal behavior due to intermolecular forces and molecular size, which are not accounted for in the Ideal Gas Law.
Applications
The Ideal Gas Law finds widespread applications in various fields:
- Chemistry:Determining the molar mass of gases, predicting gas behavior in chemical reactions
- Physics:Calculating gas pressure in containers, understanding gas expansion and compression
- Engineering:Designing gas storage systems, optimizing combustion engines
- Environmental science:Monitoring atmospheric pressure and temperature, predicting gas emissions
Understanding the Ideal Gas Law enables scientists and engineers to solve practical problems, optimize processes, and make informed decisions in fields ranging from industrial production to environmental protection.
Extensions
Further investigations to extend the study of the Ideal Gas Law could include:
- Investigating the behavior of different gases (e.g., helium, carbon dioxide) under varying conditions.
- Exploring the deviations from the Ideal Gas Law at extreme pressures or temperatures.
- Developing mathematical models to account for non-ideal gas behavior.
By extending the study of the Ideal Gas Law, researchers can gain a deeper understanding of gas behavior and its applications in diverse fields.
FAQ Section
What is the Ideal Gas Law?
The Ideal Gas Law is a mathematical equation that describes the relationship between the pressure, volume, and temperature of a gas under ideal conditions (i.e., assuming the gas particles are point masses with no intermolecular forces).
Why is the Ideal Gas Law important?
The Ideal Gas Law is important because it provides a simple and accurate way to predict the behavior of gases under a wide range of conditions. It is used in many fields, including chemistry, physics, and engineering.
What are the limitations of the Ideal Gas Law?
The Ideal Gas Law is only accurate under ideal conditions. At high pressures and low temperatures, gas particles may exhibit non-ideal behavior due to intermolecular forces. Additionally, the Ideal Gas Law does not account for the behavior of real gases at very low temperatures, where quantum effects become significant.