Chemical equations gizmo answer key offers a comprehensive guide to balancing chemical equations, a crucial aspect of understanding chemical reactions. This powerful tool empowers students and educators alike to delve into the intricacies of chemical transformations, unraveling the secrets of molecular interactions.
The Chemical Equations Gizmo, an interactive simulation, provides a user-friendly interface that simplifies the process of balancing equations. With its intuitive features and step-by-step guidance, the Gizmo empowers learners to grasp the fundamental principles of stoichiometry and chemical equilibrium.
Overview of Chemical Equations Gizmo: Chemical Equations Gizmo Answer Key
The Chemical Equations Gizmo is an interactive tool that helps students understand and balance chemical equations. It allows students to enter reactants and products, and then the Gizmo automatically generates a balanced equation. The Gizmo also includes a number of advanced features, such as the ability to generate balanced equations from reactants or products, and to track the changes in mass and energy that occur during a reaction.
The Gizmo is a valuable tool for teaching and learning chemistry. It can help students to visualize the process of balancing equations, and to understand the underlying principles of chemical reactions.
Balancing Chemical Equations with the Gizmo
To balance a chemical equation using the Gizmo, students first need to enter the reactants and products into the Gizmo. They can then click the “Balance” button, and the Gizmo will automatically generate a balanced equation. The Gizmo will also show the steps involved in balancing the equation, so that students can see how the equation was balanced.
The Gizmo can be used to balance both simple and complex equations. For example, the following equation can be balanced using the Gizmo:
Fe + HCl → FeCl2+ H 2
The Gizmo will generate the following balanced equation:
Fe + 2HCl → FeCl2+ H 2
Advanced Features of the Gizmo
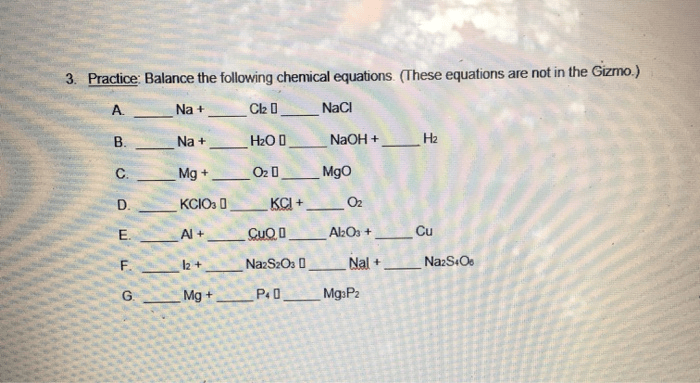
The Gizmo includes a number of advanced features that can be used to solve more complex problems. For example, the Gizmo can be used to generate balanced equations from reactants or products. This can be useful when students are given a set of reactants or products and need to determine the balanced equation for the reaction.
The Gizmo can also be used to track the changes in mass and energy that occur during a reaction. This can be useful for understanding the energetics of a reaction and for predicting the products of a reaction.
Applications of the Gizmo in Chemistry Education
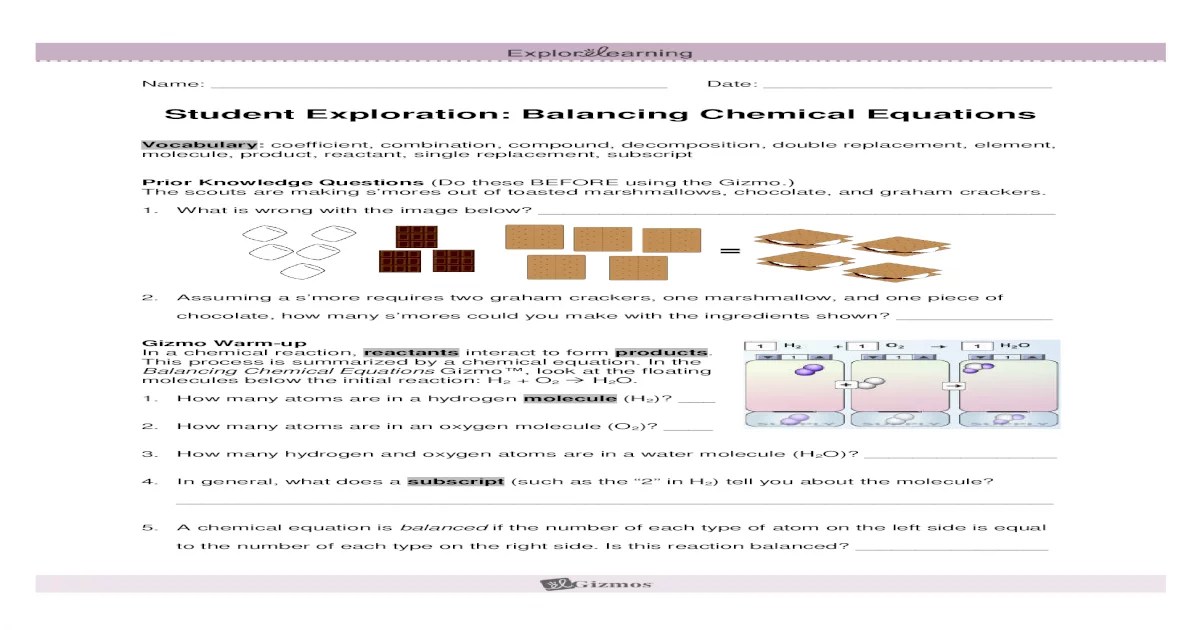
The Gizmo can be used to enhance chemistry education in a number of ways. For example, the Gizmo can be used to:
- Teach students how to balance chemical equations
- Help students to understand the underlying principles of chemical reactions
- Provide students with a tool for solving more complex problems
- Engage students in hands-on learning
The Gizmo is a valuable tool for teaching and learning chemistry. It can help students to visualize the process of balancing equations, and to understand the underlying principles of chemical reactions.
Troubleshooting and Common Errors

Students may encounter a number of common errors when using the Gizmo. For example, students may:
- Enter the reactants and products incorrectly
- Not click the “Balance” button
- Misinterpret the results of the Gizmo
To avoid these errors, students should carefully follow the instructions for using the Gizmo. They should also make sure that they understand the underlying principles of chemical reactions.
Quick FAQs
What are the benefits of using the Chemical Equations Gizmo?
The Gizmo provides an interactive and engaging way to learn about balancing chemical equations, making the process more accessible and enjoyable.
How can I use the Gizmo to balance complex equations?
The Gizmo’s advanced features allow users to generate balanced equations from reactants or products, simplifying the balancing process for complex reactions.
What are some common errors that students make when using the Gizmo?
Common errors include forgetting to balance the number of atoms of each element on both sides of the equation or failing to account for charges when balancing ionic equations.